National Programme on AMR Containment
Antimicrobial resistance (AMR) is one of the top global public health challenges of the current times which threatens the reversal of gains of modern medicine. Appropriate containment measures are required to be undertaken on an urgent basis to prevent untreatable illness from becoming a reality.
Government of India has given due cognizance to the problem of AMR and launched the “National Programme on AMR Containment” during the 12th five-year plan in the year 2013. The programme is being coordinated by the Centre for Bacterial Diseases and Drug Resistance (CBDDR), National Center for Disease Control (NCDC).
The current objectives of this programme are:
- 1Establish a laboratory-based AMR surveillance system in the country to generate quality data on antimicrobial resistance
- 2Carry out surveillance of antimicrobial usage in different health care settings
- 3Strengthen infection control practices (IPC) and promote rational use of antimicrobials through antimicrobial stewardship activities
- 4Generate awareness amongst health care providers and community on antimicrobial resistance and rational use of antimicrobials.
Activities carried out under the programme:
1. AMR Surveillance
Under the programme, for the accomplishment of first objective, National AMR Surveillance network (NARS-Net) has been established in 2013 to determine the magnitude and trends of AMR in different geographical regions of the country. The labs under NARS-Net include government medical colleges. The number of labs in the network have been expanded in a phased manner and are required to submit AMR surveillance data of nine priority bacterial pathogens of public health importance, viz.,
Staphylococcus aureus, Enterococcus species,
Klebsiella species,
Escherichia coli, Pseudomonas aeruginosa, Acinetobacter baumannii/ Acinetobacter calcoaceticus complex,
Salmonella enterica serotypes Typhi and Paratyphi,
Shigella species and
Vibrio cholerae. Among the fungal pathogens,
Candida spp, from bloodstream infections are also to be submitted to the NRL for AMR in fungal pathogens for confirmation and AFST.
Under the programme, sites are supported for maintaining essential infrastructure, reagents and consumables, manpower support for ensuring Internal quality testing and generation of quality AMR data and entry into the designated software. NCDC provides technical support to network laboratories to ensure generation of quality AMR data. Sites perform AST by disk diffusion, broth microdilution, agar dilution, and automated antimicrobial susceptibility testing systems as per the programme SOPs developed at NCDC.
National Reference Laboratories (NRL): NRL for AMR in bacterial pathogens has been established in CBDDR division at NCDC. The NRL conducts EQAS testing, confirmation of AMR Alert pathogens and phenotypic and genotypic characterisation of AMR determinants.NRL for AMR in fungal pathogens has been established in Mycology Laboratory at Vallabhbhai Patel Chest Institute, Delhi University. This NRL confirms all Candida spp. isolated from blood cultures submitted by the NARS-Net sites and the AFST conducted at the NRL is included in the National data.
Both NRLs also carry out trainings for building capacity of NARS-Net sites.
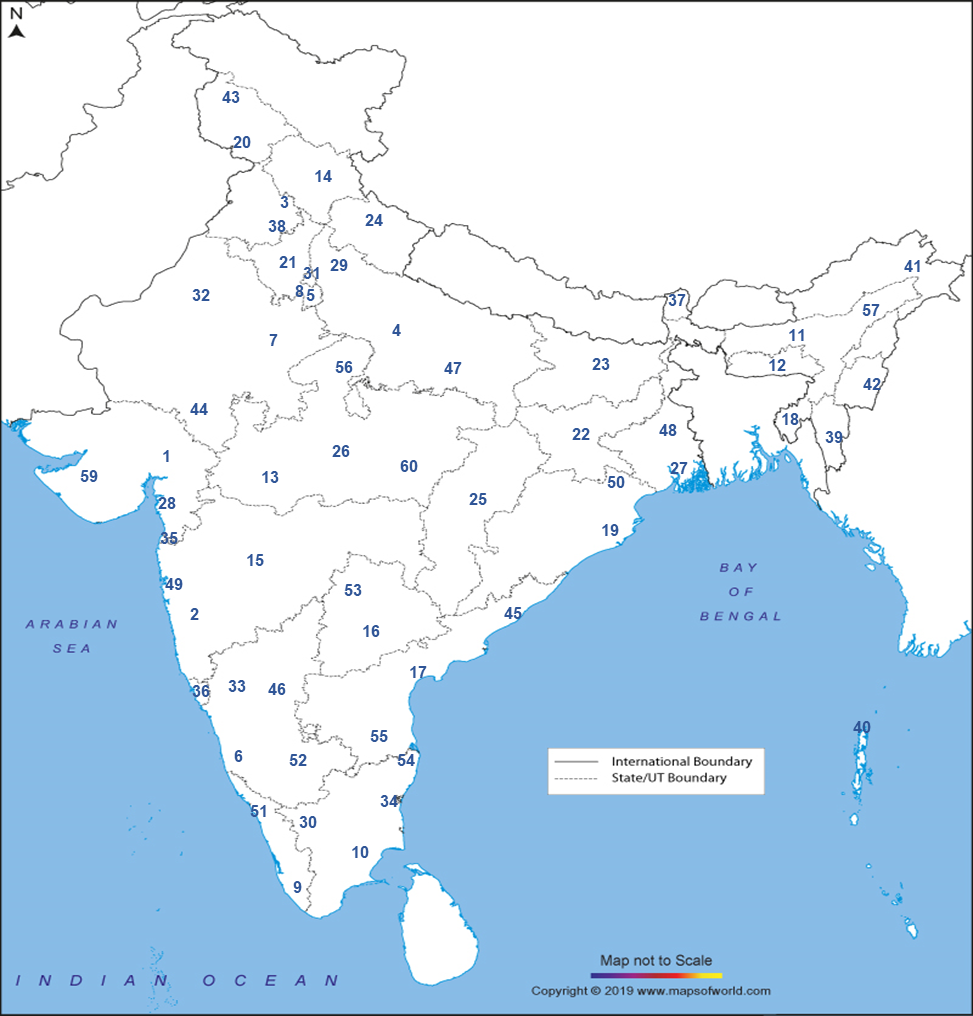
National AMR Surveillance Network (NARS-Net): The network of laboratories is being expanded in a phased manner and currently includes 60 state medical college labs in 33 States/UTs. The list of the sites in the network are as below:
- 1Lady Hardinge Medical College and Associated hospitals, Delhi
- 2Vardhman Mahavir Medical college and SJ Hospital, Delhi
- 3SMS medical College, Jaipur, Rajasthan
- 4BJ Medical College, Ahmedabad, Gujarat
- 5BJ Medical college, Pune, Maharashtra
- 6Government Medical college, Chandigarh
- 7Mysore Medical college, Mysuru, Karnataka
- 8GSVM Medical College, Kanpur, Uttar Pradesh
- 9Gauhati Medical College and Hospital, Guwahati, Assam
- 10KAPV. Government Medical College, Tiruchirappalli, Tamil Nadu
- 11NEIGRIHMS, Shillong, Meghalaya
- 12Govt. Medical College, Thiruvananthapuram, Kerala
- 13MGM College and Hospital, Indore, Madhya Pradesh
- 14IGMC, Shimla, Himachal Pradesh
- 15Govt. Medical College and Hospital, Aurangabad, Maharashtra
- 16Osmania Medical College, Hyderabad, Telangana
- 17Govt. Medical College & Hospital, Jammu, Jammu and Kashmir
- 18Agartala Govt. Medical College, Agartala, Tripura
- 19Guntur Medical College, Guntur, Andhra Pradesh
- 20SCB Medical College & Hospital, Cuttack, Odisha
- 21Pt. Jawaharlal Nehru Memorial Medical College, Raipur, Chhattisgarh
- 22Rajendra Institute of Medical Sciences, Ranchi, Jharkhand
- 23Pandit Bhagwat Dayal Sharma, Post Graduate Institute of Medical Sciences (PGIMS) Rohtak, Haryana
- 24Indira Gandhi Institute of Medical Sciences, Sheikpura, Patna, Bihar
- 25Govt. Medical College, Haldwani, Uttarakhand
- 26Gandhi Medical College, Bhopal, Madhya Pradesh
- 27Calcutta School of Tropical Medicine, Kolkata, West Bengal
- 28Lala Lajpat Rai Memorial (LLRM) Medical College, Meerut, Uttar Pradesh
- 29GMERS Medical College and Civil Hospital, Valsad, Gujarat
- 30Coimbatore Medical College & Hospital, Coimbatore, Tamil Nadu
- 31Karnataka Institute of Medical Sciences (KIMS), Hubli, Karnataka
- 32Indira Gandhi Medical College & Research Institute (IGMC & RI) Puducherry
- 33NAMO Medical Education and Research Institute (MERI), Silvassa, Dadra & Nagar Haveli
- 34Maulana Azad Medical College (MAMC) and Associated Hospitals, Delhi
- 35Sardar Patel Medical College (SPMC) and Hospital, Bikaner, Rajasthan
- 36Goa Medical College & Hospital, Bambolim, Goa
- 37STNM Medical College & Hospital, Gangtok, Sikkim
- 38Government Medical College, Patiala, Punjab
- 39Zoram Medical College, Falkawn, Mizoram
- 40Andaman & Nicobar Islands Institute of Medical Sciences (ANIIMS), Andaman & Nicobar Islands
- 41Rabindranath Tagore Medical College, Udaipur, Rajasthan
- 42Toma Riba Institute of Health and Medical Sciences, Naharlugan, Arunachal Pradesh
- 43Jawahar Lal Nehru Institute of Medical Sciences (JNIMS), Manipur
- 44Govt. Medical College Srinagar Jammu and Kashmir
- 45Andhra Medical College Vishakhapatnam, Andha Pradesh
- 46Vijayanagar Institute of Medical Sciences Ballari, Karnataka
- 47Moti Lal Nehru Medical College Allahabad, Uttar Pradesh
- 48Burdwan Medical College & Hospital Burdwan, West Bengal
- 49Grant Govt Medical College & Sir JJ Group of Hospitals, Byculla, Mumbai
- 50Pt. Raghunath Murmu Medical College & Hospital Baripada, Odisha
- 51Government Medical College, Thrissur, Kerala
- 52Bangalore Medical College and Research Institute, Bengaluru, Karnataka
- 53Kakatiya Medical College, Warangal, Telangana
- 54Madras Medical College, Chennai, Tamil Nadu
- 55S.V medical College, Tirupati, Andhra Pradesh
- 56Gajra Raja Medical College, Gwalior, Madhya Pradesh
- 57Jorhat Medical College and Hospital, Jorhat, Assam
- 58University College of Medical Sciences & GTB Hospital, Delhi
- 59Pandit Dindayal Upadhyay Medical College, Rajkot, Gujarat
- 60Netaji Subash Chandra Bose Medical College, Jabalpur, Madhya Pradesh
AMR Programme Unit at NCDC has developed programme SOPs, which are updated on regular basis, and all the sentinel site laboratories are provided training on the use of these SOPs. The SOPs developed are listed below and are also available on NCDC website
(https://ncdc.mohfw.gov.in/programme-sops/):
- 1. AMR Surveillance SOP 2023 version 2: This included 8 chapters
- • Chapter 1: AMR Surveillance in Priority Bacterial Pathogens under NARS-Net
- • Chapter 2: Broth microdilution colistin susceptibility test for aerobic Gram-negative bacteria
- • Chapter 3: Broth microdilution (BMD) vancomycin susceptibility test for aerobic Gram-positive cocci
- • Chapter 4: Colistin agar test for colistin resistance for Enterobacterales and Pseudomonas aeruginosa
- • Chapter 5: Vancomycin agar screen test for Staphylococcus aures and Enterococcus species
- • Chapter 6: Internal Quality Control for disk diffusion AST
- • Chapter 7: Guidance for submission of AMR Surveillance isolate for EQAS and reporting emerging AMR Alerts
- • Chapter 8: Preservation of bacterial isolates/ Control strains
- 2. SOP for WHONET data entry and data reporting
- 3. Tools for developing facility level antibiogram
AMR surveillance data is been submitted by the network labs to NCDC using the WHONET software on a monthly basis and data quality monitoring online calls are done with the sites to improve the quality of data and its adherence to programme SoP. The revised corrected data received from the network sites is validated and analysed at AMR Programme Unit, CBDDR, NCDC and compiled in the form of an annual reports and semi-annual bulletin. The annual report of last 7 years (from 2017- 2023) are available on NCDC website at: https://ncdc.mohfw.gov.in/reports/
National AMR Data Submission on Global AMR Surveillance and System (GLASS): India enrolled for GLASS in the year 2017 and NCDC is recognized as the national coordinating centre for submitting national AMR Surveillance data on GLASS portal every year. Since 2018, AMR Programme Unit at NCDC has been uploading National AMR surveillance data annually onto GLASS.
External Quality Assessment Scheme:The quality of data submitted under the National AMR surveillance network is ensured through External Quality Assessment Scheme (EQAS) conducted by NCDC, under which all network sites submit isolates every quarter (as per programme guidelines) to the National Reference Laboratory (NRL) established at NCDC. The AMR surveillance network sites are also mandated to submit AMR alert strains for confirmation to NRL at NCDC as and when isolated. The labs under the programme are also required to enrol onto the IAMM EQAS programme.
Trainings & workshops:
- The Network labs are provided training on WHONET software for data management. The network labs are also trained on developing antibiograms at facility level using WHONET and antibiogram development tools prepared by AMR Programme unit at NCDC are available at https://ncdc.mohfw.gov.in/programme-sops/. The macros for developing antibiogram at facility level are also developed and shared with network sites.
- All the Network labs are provided training on Broth microdilution testing for colistin and vancomycin. Since 2022, the labs are also being trained on agar dilution method for colistin AST.
- Capacity building on standardisation of basic procedures in Bacteriology including bacterial identification across the network was conducted during 2021-23 using the virtual platform (iECHO platform) in collaboration with CDC, ASM and ECHO-India.
- The AMR Surveillance priority pathogen identification modules including algorithms for identification have been developed by group of AMR Committee of Experts.
- Other activities carried out for quality improvement include onsite support visits, onsite trainings. During onsite visits, the lab capacity is assessed and hand holding is done for strengthening Internal Quality Control and Proficiency testing in network labs.
Review meetings:Annual Review meetings are conducted to review the status and site-wise progress of the network labs under the programme. During the meeting, the sites facing challenges in working and carrying out activities under AMR Surveillance are discussed in order to make AMR Surveillance robust.
2. Surveillance of Antimicrobial consumption/use:
Surveillance of Antimicrobial consumption is coordinated at selected programme sites by the Epidemiology Division and the network od these sites is named National Antimicrobial consumption networks (NAC-Net). The NAC-Net published their report in July 2023 which is available on NCDC website at: https://ncdc.mohfw.gov.in/wp-content/uploads/2024/12/NAC-NET_report-on-antimicrobial-consumption.pdf.
3. National Treatment Guidelines:
A common unified National Treatment Guidelines for antimicrobial use in infectious diseases have been released in 2016 and uploaded on the website at:
https://ncdc.mohfw.gov.in/guidelines-resources/. It is to serve as a guide to all the hospitals to formulate their own guidelines on basis of which physicians will be trained. The guidelines are being updated currently.
4. Infection Prevention & Control guidelines and surveillance of healthcare associated infections:
• “National Guidelines for Infection Prevention and Control in Healthcare facilities” have been developed with support from WHO and have been disseminated to various stakeholders and also unloaded on NCDC website at https://ncdc.mohfw.gov.in/guidelines-resources/.
• 50 sites under the National Programme on AMR Containment have been enrolled for the surveillance of healthcare associated infections. The definitions, SoPs and the portal of the ICMR-AIIMS Healthcare Associated Infections (HAI) surveillance project are being used with support from AIIMS team. For more details on ICMR-AIIMS HAI surveillance network, refer to https://www.haisindia.com/.
4. IEC Activities:
Material for IEC has been developed in phased manner and is available on NCDC website at: https://ncdc.mohfw.gov.in/iec-on-amr/. In addition, various IEC activities are conducted/coordinated round the year including CMEs in professional organisations, quiz competition in schools, public lectures in academic Institutions and radio programmes, participation in Health fairs etc.
5. Support establishment of State AMR Surveillance Network
One NARS-Net centre in each state is identified as nodal centre for the respective state for establishing state AMR surveillance networks. Under the programme through the nodal centre, sensitisation workshops and trainings are supported for medical colleges in the respective state to submit AMR data as a part of state AMR surveillance network. The nodal centre is supported to clean, analyse AMR surveillance data to generate annual surveillance reports. The state AMR Surveillance networks have been established/initiated in Maharashtra, Kerala, Delhi, Gujarat, Rajasthan, Karnataka, Madhya Pradesh, Telangana and Assam.










